Research
Transcriptional Regulation of Metabolism by Nuclear Receptors
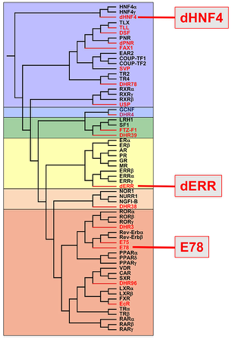
Nuclear receptors are a family of ligand-regulated transcription factors that control gene expression in response to binding small lipophilic compounds, such as fatty acids, sterols and bile acids. We use the fruit fly, Drosophila, as a simple model system to characterize the regulation and function of nuclear receptors with a focus on their role in metabolism. The Drosophila genome encodes 18 canonical nuclear receptors, compared to 48 in humans and 284 in C. elegans, providing the smallest complete set of nuclear receptors in any genetic model system. In spite of this small number, the fly nuclear receptors represent all of the major mammalian subclasses and include orthologs of key human nuclear receptors, providing a good model for studying nuclear receptor regulation and function (Figure 1). Current work is focused on three fly receptors that play central roles in metabolism: dHNF4, dERR, and E78.
dHNF4
Our past studies of dHNF4 during larval stages demonstrated a role for this receptor in an essential metabolic function - the adaptive response to starvation. In this context, dHNF4 directly induces the genes that drive fatty acid oxidation for energy production, allowing the animal to survive periods of nutrient deprivation. Our current work is focused on the essential roles of dHNF4 later in development. We discovered that dHNF4 mutant adults are hyperglycemic due to reduced levels of glucose-stimulated insulin secretion from their insulin-producing cells accompanied by decreased levels of glucose uptake by the fat body. Remarkably, these phenotypes are similar to those seen in human patients carrying HNF4 mutations - an early onset form of type 2 diabetes called MODY1. Although many attempts have been made to model MODY1 in mice, none of these were successful. Our fly model for MODY1 thus provides, for the first time, a genetic model to study the origins of this human disorder. To find out more, see our paper on dHNF4 in eLife. dHNF4 mutant adults are also sensitive to a sugar diet, have a reduced lifespan, and display a large-scale inflammatory response. The basis for these phenotypes remains unknown. Our current studies are focused on the molecular mechanisms by which dHNF4 contributes to these pathways.
dERR
Our studies of the Drosophila Estrogen-Related Receptor (dERR) have revealed that it directly activates a transcriptional program in embryos, coordinately inducing genes that play central roles in glycolysis, the pentose phosphate pathway, and lactate production. Combined with metabolomic analysis, this work indicated that dERR establishes a metabolic state related to the Warburg effect, which is normally associated with proliferating cancer cells. Drosophila undergo a dramatic increase in mass during larval development, leading to the model that dERR establishes a metabolic state during embryonic development that supports subsequent larval growth. This work shows, for the first time, that a proliferative metabolic program is used to support normal developmental growth. It also provides a model that clarifies our understanding of the close association between mammalian ERR family members and cancer. See our Cell Metabolism paper on dERR to learn more. Our current studies of dERR have moved from the larval to adult stage, when no growth occurs. Our hypothesis is that dERR adopts new functions in this different developmental context. Consistent with this, we have found that dERR mutant adults have almost no stored lipid - a phenotype we have never seen before. Our current studies are focused on determining the mechanisms by which dERR maintains proper lipid levels in the adult.
E78
We reported the isolation of the E78 nuclear receptor in a Cell paper published in 1993. Shortly after that, another study demonstrated that E78 null mutants are fully viable and fertile. Since then, the functions for this nuclear receptor have remained uncharacterized. We are currently testing the hypothesis that E78 plays a central role in metabolism similar to Its closest homologs in mammals, PPAR and Rev-erb.